Discover the Facts About the Smallest Atom Hydrogen – Wellness Group
Did you know that over 1 million hydrogen atoms could fit across the width of a single grain of sand? This tiny powerhouse, the lightest element in the universe, holds secrets that shape everything from stars to the water we drink. Wellness Group is here to unpack the science behind this fascinating topic while connecting it to real-world applications.
At its core, every atom consists of a nucleus surrounded by electrons. The distance between these particles defines its size, known as the atomic radius. For hydrogen, this measurement is remarkably compact due to its single proton and electron pairing. Recent studies highlight how its simplicity makes it a cornerstone of modern physics and chemistry.
Wellness Group bridges scientific curiosity with practical insights. Their team explains complex concepts, like how effective nuclear charge influences atomic dimensions, in easy-to-grasp terms. For those eager to dive deeper, their hydrogen-infused wellness solutions showcase the element’s versatility beyond textbooks.
This article explores hydrogen’s unique properties, compares it to helium and hydride ions, and reveals why its size matters. Readers will also learn how to connect with Wellness Group’s experts via WhatsApp at +60123822655 during specified service hours for personalized guidance.
Key Takeaways
- Hydrogen’s atomic radius is the smallest due to its single proton and electron structure.
- The nucleus and electron interactions determine an atom’s physical characteristics.
- Periodic trends help compare hydrogen’s size to elements like helium.
- Wellness Group combines scientific expertise with accessible service options.
- Effective nuclear charge plays a critical role in atomic behavior.
- Hydrogen’s simplicity makes it a foundational subject in physics research.
Introduction to Atomic Fundamentals and Wellness Group
Understanding atomic structure unlocks answers to how matter behaves. Every element consists of a nucleus packed with protons and neutrons, surrounded by electrons in motion. These tiny particles define physical properties, from conductivity to reactivity.

Overview of Atomic Structure
Electrons orbit the nucleus in specific energy levels called shells. The number of protons determines an element’s identity, while neutrons stabilize the nucleus. For example, elements with one proton and one electron exhibit unique bonding behaviors.
Atomic radius—the distance from the nucleus to the outermost shell—varies based on electron shielding and effective nuclear charge. Inner shells reduce the pull of the nucleus on outer electrons, affecting size across the periodic table.
Role of Atoms in Modern Physics
Atoms are the building blocks for breakthroughs in energy and materials science. Researchers study how charges interact within shells to develop technologies like semiconductors. Concepts like ionic radii help predict how elements combine in reactions.
About Wellness Group and Service Hours
Wellness Group bridges complex science with everyday wellness. Their team answers questions about atomic principles and their health applications. Reach them via WhatsApp at +60123822655 during service hours:
- Mon-Fri: 9:30 am–6:30 pm
- Sat-Sun: 10 am–5 pm
Stay tuned as we explore how these fundamentals apply to specific elements in the next sections.
Understanding the Smallest Atom Hydrogen
Exploring atomic dimensions reveals fascinating scientific insights. How do interactions between particles determine an element’s physical traits? Let’s break down the forces shaping these tiny structures.
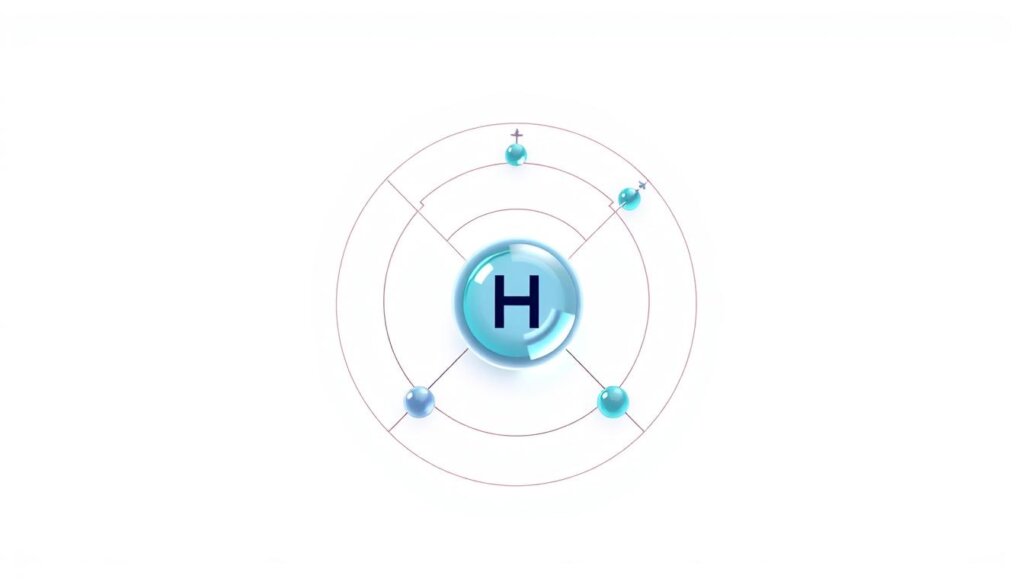
Atomic Radius, Nuclear Charge, and Shielding Effects
The atomic radius measures the distance from a nucleus to its outermost electrons. For elements with one proton and one electron, this distance depends on two factors: nuclear charge and shielding. A stronger positive charge pulls electrons closer, shrinking the radius.
Shielding occurs when inner electrons block outer ones from feeling the nucleus’ full pull. Since hydrogen lacks inner shells, its electron experiences maximum nuclear attraction. This explains its compact size compared to ions with added electrons.
Comparing Hydrogen, Helium, and the Hydride Ion
Helium has two protons and two electrons. Its higher nuclear charge outweighs shielding effects, resulting in a smaller radius (31pm) than hydrogen (53pm). When hydrogen gains an electron to form H⁻, repulsion between electrons expands its radius significantly.
Here’s how they stack up:
- Hydride ion (H⁻): 208pm radius
- Hydrogen: 53pm radius
- Helium: 31pm radius
This size hierarchy impacts how these particles bond and react. Wellness Group’s experts use such principles to explain real-world applications, from energy storage to material science. Need answers? Contact them via WhatsApp during service hours for personalized guidance.
Scientific Insights on Atomic Trends and Measurements
Patterns in the periodic table reveal hidden connections between elements. By studying how atomic radius changes across rows and down columns, scientists predict chemical behaviors with surprising accuracy.
Periodic Table Trends Explained
Moving left to right across a period, atoms shrink in size. Why? Each element gains one proton while electrons fill the same shell. The rising nuclear charge pulls electrons tighter, reducing the radius. For example, lithium (182pm) is nearly three times larger than fluorine (64pm) in period 2.
Descending a group adds new electron shells. Sodium sits below lithium and has an extra shell, making its radius 186pm versus lithium’s 182pm. This pattern explains why heavier elements often react more vigorously.
Significance of Electron Shells and Ionic Radii
Electron shells act like layered shields. Inner electrons block outer ones from feeling the nucleus’ full pull. When atoms gain or lose electrons to become ions, their radius changes dramatically. Sodium loses an electron to form Na⁺, shrinking from 186pm to 95pm.
Chlorine gains an electron to become Cl⁻, expanding from 79pm to 181pm. These shifts influence how ions bond in compounds like table salt. Wellness Group experts use such principles to answer questions about material properties during their service hours.
Need clarity on these concepts? Reach their team via WhatsApp at +60123822655 for friendly, jargon-free answers.
Conclusion
Atomic science bridges the gap between theory and real-world applications. The nucleus, packed with protons and neutrons, interacts with orbiting electrons to define an element’s traits. Effective nuclear charge and shielding effects shape atomic dimensions—key factors in understanding why particles behave as they do.
Shielding from inner shells reduces the pull on outer electrons, while periodic trends reveal size patterns across elements. Hydrogen’s single-proton structure gives it a distinct edge in compactness compared to helium’s tighter radius or the hydride ion’s expanded form. These differences influence bonding behaviors and material innovations.
Wellness Group transforms these insights into accessible knowledge. Their experts simplify complex concepts, offering reliable answers during convenient service hours. Whether you’re a student or professional, grasping atomic properties enhances problem-solving in fields like energy and health sciences.
Curious to learn more? Explore how tiny particles shape our world—and connect with Wellness Group via WhatsApp at +60123822655 for friendly guidance. Science becomes clearer when experts make it relatable.
FAQ
Why is hydrogen considered the simplest element?
It contains just one proton and one electron, with no neutrons. This minimal structure makes it unique in studying atomic behavior and interactions in fields like quantum mechanics.
How does nuclear charge affect an element’s size?
A stronger nuclear charge pulls electrons closer to the nucleus, reducing atomic radius. For example, helium’s higher charge makes it smaller than hydrogen despite similar electron configurations.
What role do electron shells play in determining ionic radii?
Adding shells increases distance between the nucleus and outer electrons, expanding size. Conversely, losing electrons (like the hydride ion) often leads to a smaller radius due to reduced electron-electron repulsion.
How does helium compare to hydrogen in atomic properties?
Helium has two protons and two electrons, giving it a greater nuclear charge. This results in a smaller radius than hydrogen, even though both occupy the first period of the periodic table.
What trends explain size variations across the periodic table?
Atomic radius decreases moving left to right (due to increasing nuclear charge) and increases top to bottom (from additional electron shells). These patterns help predict chemical reactivity and bonding behavior.
When can I reach Wellness Group for consultations?
Their team is available Monday to Friday, 9 AM to 6 PM EST, offering expert guidance on health-related applications of scientific principles like energy balance and molecular interactions.

Khloe Tan
Khloe Tan is a Certified Nutritionist, Corporate Wellness Trainer, and Holistic Health Specialist with over 15 years of experience in the health and wellness industry. She has delivered more than 100 talks nationwide, inspiring and educating diverse audiences on nutrition, lifestyle, and sustainable wellness. Her work has positively impacted over 3,000 lives, and she continues to champion holistic approaches to well-being in both corporate and personal settings.





